Abstract
Background and objective: Immune thrombotic thrombocytopenic purpura (iTTP) is a potentially life-threatening microangiopathic disorder resulting from inhibitory immunoglobulin (Ig) G-type antibodies that target plasma metalloprotease ADAMTS13. This leads to the accumulation of ultra-large multimers of von Willebrand factor (VWF) that occludes small arterioles and capillaries and causes end-organ damage. The way inhibitory antibodies work to inhibit ADAMTS13 is an active area of investigation and may lead to a better tool for diagnosing and treating iTTP. To determine whether inhibitory antibodies prevent substrate binding to ADAMTS13, which would increase the substrate concentration at which velocity is the half maximal (K0.5); decrease the maximal enzyme velocity (Vmax) of ADAMTS13 by affecting catalytic turnover; or both, we developed an assay using a well-characterized inhibitor of ADAMTS13 to explore this important question.
Method: A single chain fragment of the variable region (scFv4-20), derived from a human monoclonal antibody isolated from an iTTP patient by phage display with potent inhibitory effects on ADAMTS13 (Casina et al., PNAS. 2015; 112:9620-25), was expressed and purified. Recombinant full-length ADAMTS13 and native ADAMTS13 in normal human plasma (NHP) were titrated with a fluorescein-labeled surrogate substrate (FRETS-VWF73) in the presence of an increasing fixed concentration of scFv4-20. Titrations were analyzed both individually and applied to a global fit, which allowed for evaluation of the relative effects of the inhibitor on Vmax and K0.5. The relative effect of scFv4-20 on K0.5 was inferred from the global fit via the parameter α; values of α within an order of magnitude of unity suggest little to minimal effect of the inhibitor on K0.5.
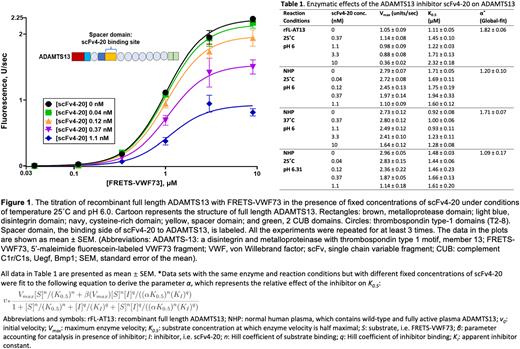
Results: When native ADAMTS13 in normal human plasma was titrated with FRETS-VWF73 in the presence of increasing concentrations of scFv4-20, the Vmax decreased with increasing concentration of scFv4-20, but the K0.5 did not show a significant change under the standard assay conditions (pH 6.0, 25 ˚C) (Fig. 1). Similar results were observed when acidic conditions closer to physiologically relevant pH and temperature were used (Table 1). Titration of recombinant ADAMTS13 with FRETS-VWF73 revealed similar results regarding the effect of scFv4-20 on the Vmax and K0.5 (Table 1) The parameter α was close to 1 when the titrations were analyzed by global fit, regardless of the reaction conditions or type of ADAMTS13 used, suggesting little to no effect of scFv4-20 on K0.5 (Table 1).
Conclusions: The antibody-mediated inhibition of ADAMTS13 by the inhibitory scFv4-20 likely results primarily from reducing catalytic turnover of the enzyme rather than the binding of ADAMTS13 to VWF. Our ongoing study is to determine whether this is a general feature of inhibitory antibodies against ADAMTS13 or if other inhibitory mechanisms are also involved in the pathophysiology of iTTP. The assay developed and described here can be applicable to explore the way antibodies affect the activity of ADAMTS13 in this polyclonal autoimmune disease.
Key words: Immune thrombotic thrombocytopenic purpura; ADAMTS13, inhibitory antibody
Disclosures
Zheng:Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Alexion: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.